Water Qualities
#012 Water Quality Standards in Agriculture
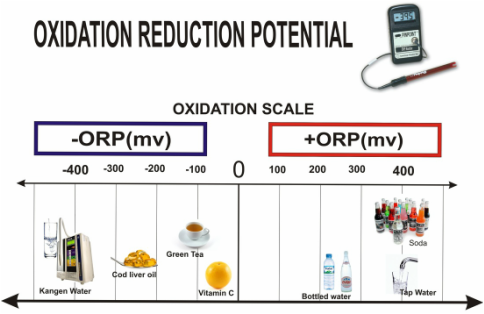
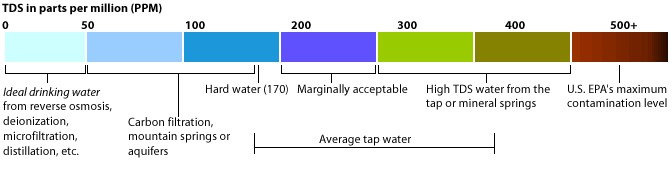
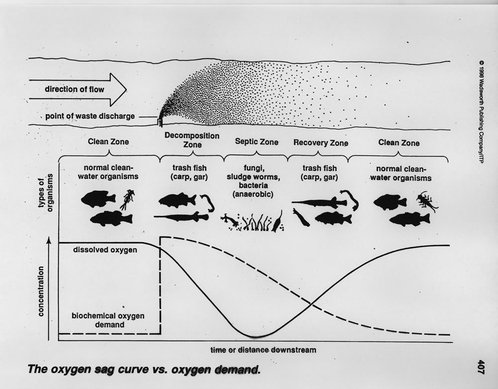
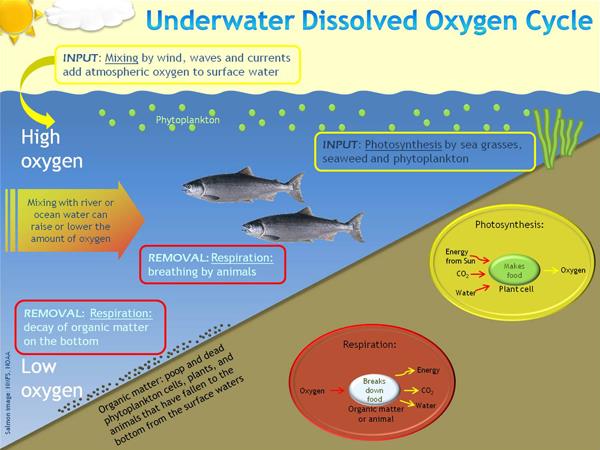
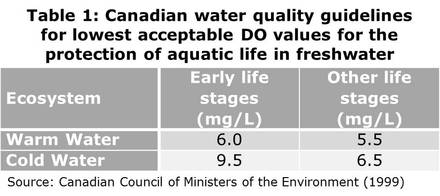
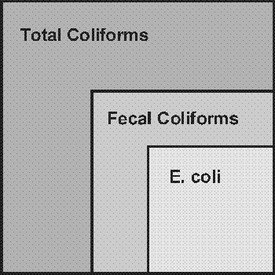
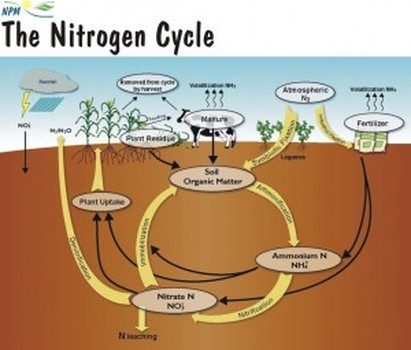
#001 Water Quality Parameters for Vegetable Washwaters
Regulatory Considerations
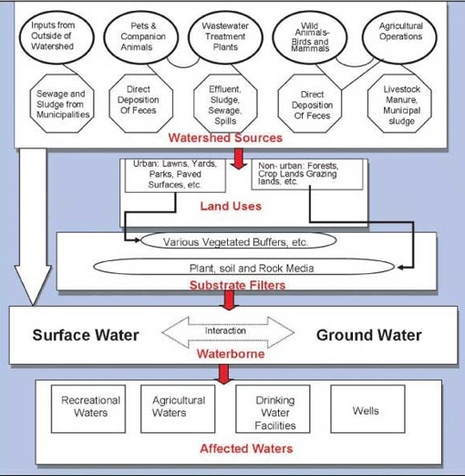
#004 Considerations when Determining Discharge Limits
#018 Selecting a Laboratory
#006 Water Sampling & Proper Procedures
#009 Regulatory Permitting & Compliance
Technology Considerations
#016 Design Considerations for Vegetable Washwater Treatment Systems
#007 Choosing Washwater or Water Treatment Technologies
#002 Impact of Muck Soils on Water Treatment Systems
Large Solid Removal
#014 Large Solid Removal for Effective Treatment
Technology Investigation: Filter Bags (available here)
#005 Settling Ponds & Tanks
#008 Drum Filters
#010 Hydrocyclones & Centrifuges
Small Solid and Nutrient Removal
#003 Coagulation & Flocculation
#013 Biofiltration
Technology Investigation: Ultrafiltration & Capacitive Deionization (available here)
Polishing and Dosing
#011 Bottom Aeration
#017 Surface Aeration
#015 Water Treatment Technology Options for Washing Vegetables

 RSS Feed
RSS Feed
