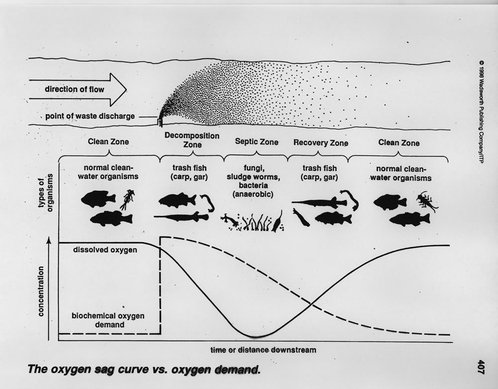
When water contains excess OM content the BOD/CBOD will be high, resulting in low dissolved oxygen (DO) concentrations; this has negative repercussions on aquatic systems. The reduction of DO is one of the main concerns when loading excess OM into a system. In aquatic systems with low DO concentrations the OM may be decomposed by anaerobic (do not require oxygen) organisms. The anaerobic decomposition of organic material produces hydrogen sulfide gas, which can be toxic to wildlife and humans. High OM loads can also cause difficulties with irrigation and plumbing due to biofilm formation and clogging, and they can reduce the efficiency of disinfection systems, which can impact food safety.
For more information on Dissolved Oxygen, see 'Defining Dissolved Oxygen'.
References
- Dorcey, A. & Freedman, B. 2013. Water Pollution. Historica Canada. http://www.thecanadianencyclopedia.ca/en/article/water-pollution/
- CCOHS. 2010. CHEMINFO: Hydrogen Sulfide. Canadian Centre for Occupational Health and Safety. http://www.ccohs.ca/products/databases/samples/CHEMINFO.html#TOC2
- Delzer, G.C. & McKenzie S.W. 2003.7.0 Five Day Biochemical Oxygen Demand. USGS TWRI Book 9–A7 (Third Edition). United States Geological Survey. Reston, VA, USA. http://water.usgs.gov/owq/FieldManual/Chapter7-Archive/chapter7.2/7.2.html
 RSS Feed
RSS Feed
